Hypothesis
The scientific community is becoming increasingly polarized regarding the role of copper in the context of heavy metal exposure (with or without it). The long-standing notion that accumulated copper is toxic to tissues seems to be slowly changing.
The broad spectrum of symptoms caused by heavy metals is primarily due to their ability to partially or completely inhibit any enzyme function or synthesis, depending on their location. One consequential manifestation of this is the increase in unbound copper levels in the liver or nervous system tissues.
This is referred to in scientific circles as “secondary copper toxicity.” (Primary toxicity occurs when someone supplements with so much exogenous copper that it causes damage to blood formation. There is no need to be overly concerned about this because it comes into play around the 20,000 mg mark, and even above 100,000, it is not necessarily fatal. RDA: 1mg)
The presence of oxidative stress is obvious, as it is a characteristic companion to heavy metal burden, yet it is often charged to the account of copper. This usually marks the beginning of the wide arc of avoidance of copper.
However, the matter limps from several directions when it comes to establishing toxicity:
8 reasons to reconsider copper supplementation
Liver biopsy
The precise distribution of copper in tissues can only be accurately determined through a rather painful liver biopsy. Who would volunteer? Exactly… not many have. The phenomenon has been studied in animals, and the ingested copper was not proportional to the values accumulated in the liver. Thus, the direct correlation between the exact amounts and the presumed toxicity is highly questionable.
Central nervous system examination
Examining the central nervous system in this manner is even more difficult. There are meticulous imaging procedures that are so expensive that a reasonable person would prefer an “autopsy of the brain.”
Post-mortem studies
Drawing conclusions from post-mortem tissue analyses about an ion involved in redox processes can be quite misleading. Put simply, living and dead tissues will yield different values.
Laboratory tests
Laboratory tests do not provide an accurate value for the amount of copper available to the body or its location. Most copper is found in the bone marrow, so current serum levels are not a definitive measure.
Ceruloplasmin & copper metabolism
The most significant copper transport protein, ceruloplasmin, which is also a copper-dependent enzyme, is inadequately produced when copper metabolism is compromised. The levels in the serum are not indicative because laboratories cannot differentiate between the copper-carrying (holoenzyme) and the substrate-less (apo-ceruloplasmin). Low levels are certainly a bad sign.
Copper toxicity?
The symptoms of copper deficiency and “copper toxicity” are suspiciously similar. Neurological symptoms, chronic fatigue, inhibited collagen synthesis, histamine reactions, skin symptoms, immunological phenomena.
Wilson’s disease
In cases of genetically disrupted copper metabolism (Wilson’s Disease), chelation techniques can remove up to 2000 mg of copper from the body daily. Why would an intake value (5/10/15 mg) cause further toxicity?
Lack of Studies
No studies have yet been conducted that aimed at long-term functional copper supplementation, neither in the presence of heavy metals nor without. Practical observations and case studies indicate that restoring copper balance with proper implementation can take weeks, and without it, even longer. According to the current accepted viewpoint, starting such a process would be toxic, thus it has not been examined on a scientific axis.
Nor has the opposite.
+1 MOST IMPORTANTLY: It helps maintain the body’s mineral balance
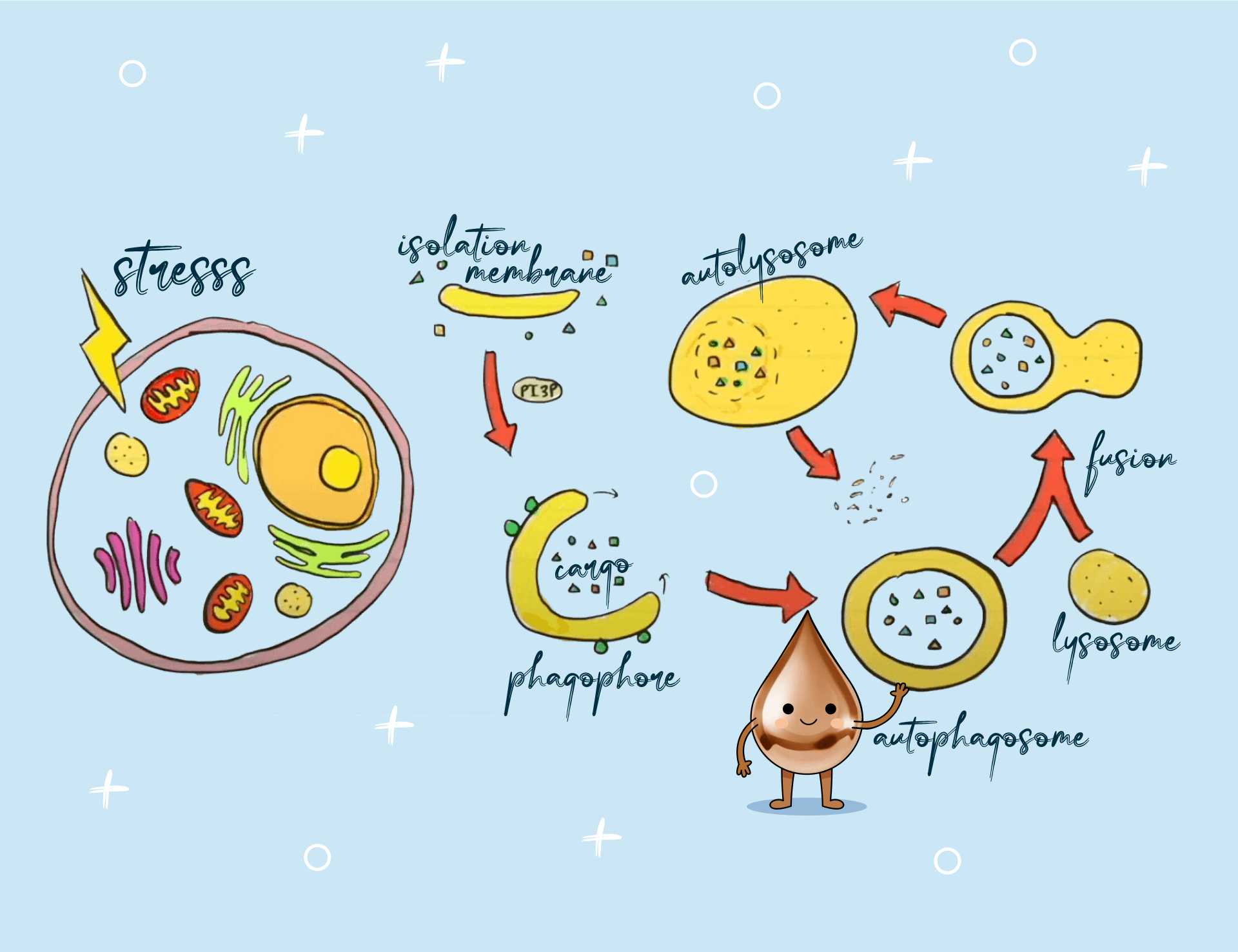
In any case of defective copper metabolism (dysregulation), enzymes such as superoxide dismutase, glutathione, ceruloplasmin, or metallothionein fail to form or function improperly.
The latter, as its name suggests, uses cysteine groups to maintain the body’s mineral balance and is responsible for the physiological resolution of heavy metal burden. Its roles in detox processes, immunological phenomena, or in controlling oxidative stress should not be overlooked either.
Conclusion
Could it be that copper is not the cause but the solution?
Could the presence of copper in tissues not be the root, but rather an indicator of existing oxidative and enzymatic issues?
Might it be that accumulated copper is the remnant of unincorporated enzymes attempting to address problematic areas where heavy metal burden (in the nervous system) and accompanying pathogens (in the liver) are most prevalent?